valence electrons in se|Selenium : Bacolod Mar 23, 2023 PDT / PST. Pacific Daylight Time . This time zone converter lets you visually and very quickly convert IST to PST and vice-versa. Simply mouse over the colored hour-tiles and glance at the hours selected by the column. and done! IST stands for India Standard Time.
valence electrons in se,The 3rd element in group-16 is selenium. The valence electrons are the total number of electrons in the last orbit(shell). The total number of electrons in the last shell after the electron configuration of seleniumis called the valence electrons of selenium. The valence electrons determine the properties of the . Tingnan ang higit paThe valence electron has to be determined by following a few steps. The electron configuration is one of them. It is not possible to . Tingnan ang higit pa
The elements that have 5, 6, or 7 electrons in the last shell receive the electrons in the last shell during bond formation. . Tingnan ang higit paThe ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). There are some . Tingnan ang higit pa
Mar 23, 2023 There are two ways to find the number of valence electrons in Selenium (Se). The first is to use the Periodic Table to figure out how many electrons Selenium has in its valence shell. To.
It has the symbol of Se and the atomic number 34. Flerovium Valence Electrons. Helium Valence Electrons. Plutonium Valence Electrons. Lithium Valence Electrons. Mercury Valence .
Learn how to determine the number of valence electrons for an element using the periodic table. An atom's valence electrons are the electrons in its outermost shell. In the .Electron configuration for selenium. The history of Selenium. Periodic table history. Identifiers. List of unique identifiers for Selenium in various chemical registry databases. .Selenium. 34. 78.971. Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. .valence electrons in se Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical . About. Transcript. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of .
Valence electrons are the outer-shell electrons of an atom. Valence electrons determine the reactivity of an atom. Atoms have a tendency to have eight . Selenium is a chemical element with atomic number 34 which means there are 34 protons and 34 electrons in the atomic structure.The chemical symbol for Selenium is Se. Electron Configuration and Oxidation States of Selenium. Electron configuration of Selenium is [Ar] 3d10 4s2 4p4. Possible oxidation states are +4,6/-2. Electron . Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd .
As a gas or vapor, the halogens all had a pungent odor. After the development of quantum mechanics, it was shown that the halogens all had seven valence electrons, supporting their original placement into .Selenium Selenium is a classified nonmetal and its symbol is ‘Se’. Selenium is the 34th element of the periodic table so its atomic number is 34. The atomic number of an element is equal to the number of protons and electrons in that element. Therefore, a selenium atom has thirty-four protons and thirty-four electrons. sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group .
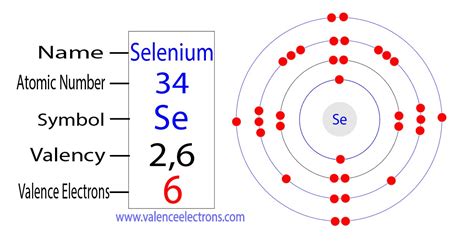
Reduced electronic configuration Se: [Ar] 3d 10 4s 2 4p 4. Below is the electronic diagram of the Selenium atom Distribution of electrons over energy levels in the Se atom 1-st level (K): 2 2-st level (L): 8 3-st level (M): 18 4-st level (N): 6. Valence electrons of Selenium. The number of valence electrons in a Selenium atom - 6. Below are .
Valency of Selenium. Selenium can hold multiple valencies in different occasions as per its compound. It may generally have the valency of -2,4 and 6. The electron configuration of this chemical element is 2-8-18-6. It clearly has the 6 electrons in its outer shell to have variable valency. Filed Under: Period Table. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable .
SeF5- lewis structure has a Selenium atom (Se) at the center which is surrounded by five Fluorine atoms (F). There are 5 single bonds between the Selenium . Total valence electrons in SeF5- ion = valence electrons given by 1 selenium atom + valence electrons given by 5 fluorine atoms + 1 more electron is added due to 1 .Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..
Generally, valence electrons can participate in the formation of chemical bonding, but core electrons cannot. While core electrons are not involved in bonding, they influence the chemical reactivity of an atom. The electron configuration of a oxygen atom is. O: 1s22s22p4 (1.9B.1) (1.9B.1) O: 1 s 2 2 s 2 2 p 4. which may be shorted. In the above electron configuration, the highest energy level (4) is marked with green color. The 4 th energy level contains 4s and 4p subshells. There are 2 electrons in the 4s subshell and 4 electrons in .Valence electrons are responsible for the reactivity of an element. They determine how "willing" the elements are to bond with each other to form new compounds. If the valence shell of an element is full, such as with a .

When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. For example, fluorine has seven valence electrons, so it is most .
SeS3 lewis structure has a Selenium atom (Se) at the center which is surrounded by three Sulfur atoms (S). There are 3 double bonds between the Selenium atom . Total valence electrons in SeS3 molecule = valence electrons given by 1 selenium atom + valence electrons given by 3 sulfur atoms = 6 + 6(3) = 24. Step 2: .valence electrons in se Selenium Helium is an exception that has only two valence electrons, but they are shown paired. Figure 2.6.1 2.6. 1: Lewis symbols or electron-dot symbols of the first twenty elements in the periodic table. The electron dots in the Lewis structure are a convenient way to determine how many bonds an atom of an element can make.

Selenium consists of 34 electrons distribution in its 4 orbits. So electronic configuration of selenium define as: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 3d10 4p 4. Or. The electronic configuration can also be represented with the help of a full electron distribution element that is Argon/ Ar. In this case it is written as [Ar] 3d 10 4s 2 4p 4.
Step #1: Calculate the total number of valence electrons. Here, the given molecule is SeOF2. In order to draw the lewis structure of SeOF2, first of all you have to find the total number of valence electrons present in the SeOF2 molecule. . (Se) atom = 6 Valence electrons given by Oxygen (O) atom = 6 Valence electrons given by each .
valence electrons in se|Selenium
PH0 · Valence electrons (video)
PH1 · Valence Electrons Chart for All Elements
PH2 · Selenium Valence Electrons
PH3 · Selenium (Se)
PH4 · Selenium
PH5 · How to Find the Valence Electrons for Selenium (Se)?
PH6 · How to Find the Valence Electrons for Selenium (Se)
PH7 · Determine valence electrons using the periodic table
PH8 · 3.1: Valence Electrons
PH9 · 10.6: Valence Electrons